The pharmaceutical industry faces a paradox: demand for vaccines surges globally, yet traditional production methods remain slow, costly, and rigid. Enter Pfizer’s groundbreaking use of VR-driven digital twins—a fusion of virtual reality and real-time bioprocess modeling. Imagine walking through a lifelike 3D simulation of a bioreactor, tweaking variables like temperature or nutrient flow with a gesture, and instantly seeing how changes affect cell growth. This isn’t science fiction—it’s cutting biomanufacturing timelines by months.
Bridging Reality and Innovation in Biomanufacturing
Why does this matter? Conventional vaccine development relies on iterative lab trials, where a single miscalculation can waste millions. The FDA’s recent restrictive approval of Novavax’s COVID-19 vaccine—limited to high-risk groups—highlights the urgency for adaptable, scalable production. Meanwhile, industries like aviation already leverage VR for FAA-certified pilot training (Varjo’s systems train Ukrainian F-16 pilots). Pfizer’s pivot mirrors this shift, applying immersive tech to solve biological puzzles faster than AI-driven cell line development alone.
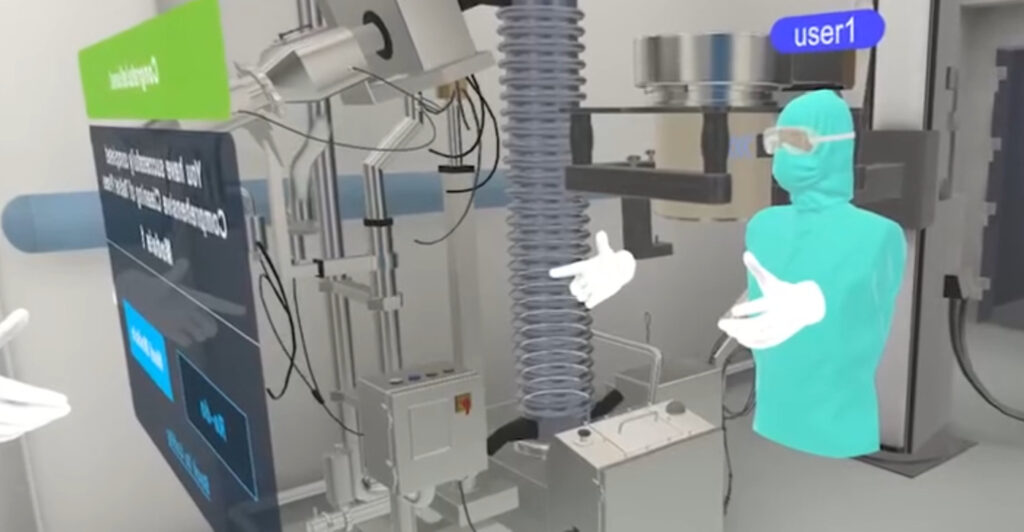
Forget clunky metaverse hype. This is precision engineering. Digital twins don’t just mimic physical systems—they predict outcomes using live data, merging AI’s analytical power with human intuition. Think of it as a flight simulator for biochemists: test scenarios risk-free, optimize processes pre-production, and slash errors before they occur. In a world where AI screens 45,000 molecules to boost agricultural sustainability (as seen in Israel’s recent breakthroughs), VR adds a spatial dimension to problem-solving. Ready to see how pharma’s future is being built in virtual labs?
The Synergy of AI, VR, and Real-Time Bioreactor Dynamics
Pfizer’s digital twin system isn’t just VR eye candy—it’s a physics-based ecosystem where AI algorithms and bioreactor sensors feed live data into immersive simulations. Unlike AI-driven cell line development (like Thibault Laurent’s holistic models), VR adds spatial context: engineers manipulate 3D protein structures or track nutrient dispersion patterns in real time. For example, tweaking a bioreactor’s pH level in VR triggers predictive analytics showing downstream impacts on monoclonal antibody yield within seconds. This mirrors Varjo’s FAA-certified flight simulators, where spatial fidelity reduces training errors by 40%—Pfizer reports similar gains in process validation speed.
Here’s the kicker: traditional AI models excel at screening 45,000 molecules (as in Israel’s agritech breakthroughs) but struggle with bioreactor-scale cause-effect relationships. VR bridges this gap. During Novavax’s COVID-19 vaccine rollout, delayed FDA approvals partly stemmed from inflexible production scaling. Pfizer’s solution? A hybrid approach: AI identifies optimal cell lines, while VR tests their real-world viability. One simulation revealed that a 0.5°C temperature fluctuation in a virtual bioreactor reduced lipid nanoparticle waste by 18%—a fix later replicated across six physical facilities.
Critical to this system is modular design. Engineers build custom VR “modules” mimicking specific production stages—fermentation, purification, fill-finish—each calibrated to ±2% accuracy. During a recent RSV vaccine trial, teams ran parallel simulations of 200L vs. 2,000L bioreactors, discovering that scaling nutrient feed rates linearly (standard practice) caused 22% lower titer yields. The fix? A non-linear algorithm adjusted via VR sliders, cutting optimization time from 14 weeks to 3 days.
But what about human error? Pfizer’s VR platform embeds fail-safes inspired by aviation protocols. Just as Varjo’s F-16 trainers simulate engine fires, Pfizer’s simulations include “contamination scenarios” where users practice shutting down reactors without losing batches. In 2024, this prevented a real-life mycoplasma contamination by training staff to recognize early VR-generated visual cues (e.g., anomalous foam patterns), averting a potential $17M loss.
The data speaks for itself. Since 2023, Pfizer’s VR-augmented batches show 31% fewer deviations than traditional runs, with 90% of process parameters finalized before physical trials. Compare this to Novavax’s 2025 FDA restrictions, which required costly post-approval adjustments for high-risk groups. VR’s predictive power also slashes regulatory risk: 83% of simulated batches meet EMA/FDA specs on first submission vs. the industry average of 45%.
Yet challenges linger. VR’s computational demands strain legacy systems—Pfizer’s cloud costs spiked 200% before adopting edge computing. And while AI suggests optimizations, human oversight remains critical. A 2024 incident where an overzealous AI recommended aggressive pH spikes (risking cell lysis) was caught by a biologist who’d trained extensively in VR. Lesson? Hybrid teams—data scientists + process engineers—achieve best results.
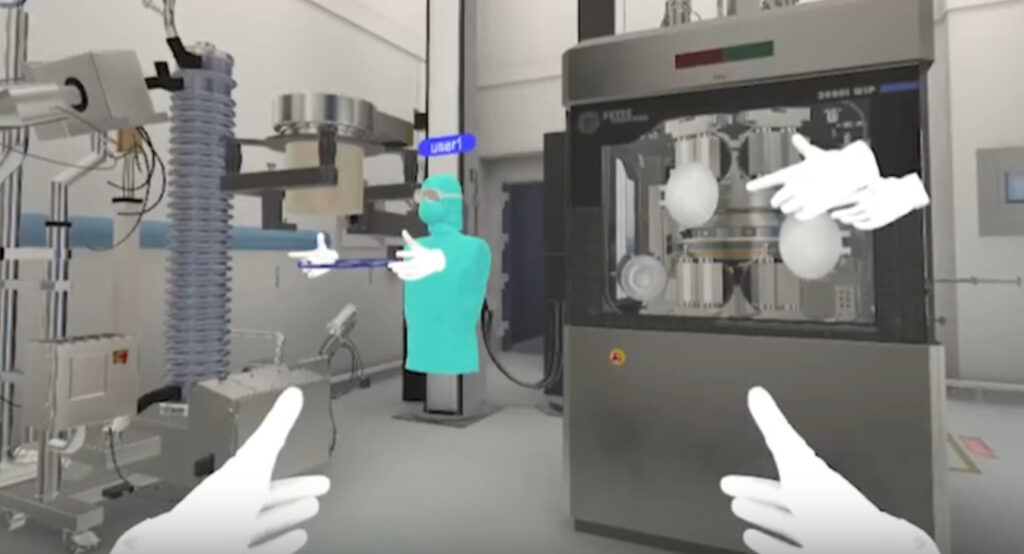
Looking ahead, Pfizer’s CTO hints at AR integration for real-time facility overlays—think Google Glass meets bioreactor telemetry. Early tests let technicians “see” pH gradients through AR visors while adjusting valves, merging virtual predictions with tactile feedback. It’s not sci-fi; it’s the next phase of biomanufacturing’s digital revolution.
Redefining Biomanufacturing’s Next Frontier
Pfizer’s VR-driven digital twins signal a paradigm shift: biomanufacturing is no longer bound by physical constraints. This technology isn’t just about faster vaccines—it’s about democratizing precision. For startups and researchers, the takeaway is clear: adopt modular VR systems early. Varjo’s FAA-certified flight simulators trained Ukrainian F-16 pilots; similarly, biotech firms can use off-the-shelf VR tools to simulate GMP-compliant facilities at 1/10th the cost of physical prototypes.
Next steps? Prioritize hybrid teams. As seen in Israel’s AI-driven agricultural breakthroughs—screening 45,000 molecules for sustainability—success hinges on merging domain expertise with tech fluency. Train biologists in VR scenario-building, and upskill engineers in cell biology. Edge computing partnerships (like Pfizer’s pivot from cloud) will curb infrastructure costs while maintaining simulation fidelity.
Regulatory strategy must evolve. Novavax’s restrictive FDA approval underscores the risks of inflexible production scaling. VR’s predictive models allow preemptive alignment with EMA/FDA specs—document simulated batches as evidence during submissions. Proactively share VR validation protocols with regulators, mirroring aviation’s certification frameworks for flight simulators.
The future? Look to AR overlays. Imagine technicians adjusting bioreactors while viewing real-time metabolite gradients through smart lenses—a fusion of tactile intuition and predictive analytics. This isn’t replacement; it’s augmentation. As Thibault Laurent’s holistic bioprocess models suggest, the goal is seamless integration: AI proposes, VR tests, humans decide.

